Pig Model of Neural Injury
Our Research
Currently, our lab is developing two pig models for translational research. In order to address the lack of current treatments after stroke in adult humans, we are developing a pig stroke model using adult pigs. We hope that by introducing neural stem cells into the injury site after a stroke, we will be able to regenerate the lost or damaged tissue and provide functional recovery. In addition, in order to address the lack of current treatments after an toddler or young child has sustained a concussive traumatic brain injury, we are developing a piglet concussive traumatic brain injury model. We hope that by introducing neural stem cells into the injury site after a traumatic brain injury, we can regenerate the lost of damaged tissue and provide functional recovery.
Current Challenges using other models?
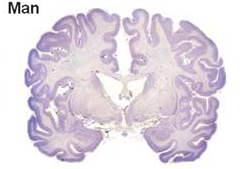
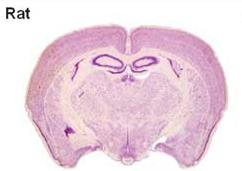
Currently, most treatments for neural injuries are being developed by utilizing rodent models. Unfortunately, the human brain differs greatly from the rodent brain in size, architecture, and composition , therefore treatments developed on rodent models do not always translate successfully to humans. For example, a human brain is 750 times larger than a rodent brain. Not only is the rodent brain substantially smaller than the human brain, but the types of cells found within the brain are also substantially different. As a whole, the composition of a rodent brain is made up of white and gray matter, similar to a human brain; However a rodent brain is composed of much lower levels of white matter as compared to humans To put it into perspective, the human brain is composed of ~60% white matter, while the rodent brain is composed of ~10% white matter. Different treatments have different effects on white and gray matter, thus it’s easy to see why so many treatments tested on rodents are ineffective for humans.
(S. Herculano-Houzel, Human Neuroscience, 2009)
Why is the pig model better?
Due to the challenges of using rodents models, we looked to find a model whose brain more closely resembled a human brain. We choose to use a pig as our model for several key reasons. First, the human brain is only 7.5 times the size of an average pig brain. The size of the brain is important in our study because we want to be able to translate potential treatments directly to humans. Second, the human brain is gyrencephalic, meaning that outer cerebral cortex of the brain has many convolutions, while the rodent brain is lissencephalic, meaning that the outer cerebral cortex of the brain is smooth. Typically a brain that has more convolutions (i.e. gyrencephalic) is associated with higher connectivity and complexity. The pig brain is similar to humans in that it has a gyrencephalic brain again making it an easily translatable model. Finally, the pig has a similar white to gray matter ratio within the brain as compared to humans (60:40 white to gray matter). Thus if we inject neural stem cells into a pig brain, we would expect recovery of both white and gray matter to be similar in pigs and humans.
(Howells, D.W., et. al, Journal of Cerebral Blood Flow and Metabolism, 2010)
What can we learn using this model?
As of now, few therapies have been approved for use after a neural injury to the brain. This pig model will help us bridge the gap between the current translational failures of treatments developed using rodent models for human therapies. Presently, over 700 drugs have gone to clinical trial for stroke alone, but only one drug has been FDA approved- Tissue Plasminogen Activator (t-PA)- which has had limited success. Thus it is our hope that the development of a pig model will allow for more stringent assessment of the efficacy and safety of novel cell therapies that can potentially be translated to humans with greater success.